Semiconductors are critically important in our world. The most significant application is in computerized or radio waves-powered electrical devices. These semiconductors are made of the widely abundant element, silicon. In fact, silicon is at the core of almost all kinds of electronic devices.
One of the reasons why they are widely used in semiconductors is because they are abundant in nature. For example, silicon occurs naturally in sand and quartz, which has a perfect electronic structure. The use of silicon in semiconductors explains why Silicon Valley is the home of many tech companies.
Another very important thing to note about silicon is the impact of doping, a process in which impurities are introduced to enhance the value of an intrinsic semiconductor. Read on to learn more details about doping in silicon semiconductors.
Structure of Silicon
Just like carbon, silicon has 4 electrons in its outer orbital, which contributes to its crystalline structure. Silicon has the appearance of a silvery, metallic substance. However, silicon is not a metal, even though its crystal assumes a metallic structure. In fact, silicon acts as an insulator, allowing only a small amount of electricity to pass through it.
It is neither a good insulator nor a good conductor, hence the name semiconductor. When other elements like phosphorus or boron are introduced into the crystalline structure of silicon, then we have either an n-type or p-type semiconductor.
Types of Semiconductors
Semiconductors are generally materials or elements that behave like normal conductors and also act like typical insulators. However, they cannot be said to be strong conductors or insulators. In practical applications, semiconductors fall into two types of categories, as discussed below.
Intrinsic Semiconductors
Intrinsic semiconductors are made up of only one kind of material, examples of which can either be silicon or germanium. Another name for this type of semiconductor is undoped semiconductors or i-type semiconductors. These are chemically pure semiconductors. In these semiconductors, electrons have energies in only certain bands. The energy bands correspond to a large number of discrete quantum states of electrons. Also, note that the distribution and population of bands in semiconductors is another factor that distinguishes them from metals.
For clarity, the valence band of electrons in metals is typically almost filled under normal conditions, but in semiconductors, only a few electrons exist in the conduction band, just above the valence band. Insulators, on the other hand, have nearly no free electrons, which is why they can’t conduct electricity. Also, in intrinsic semiconductors, the number of holes is equal to the number of excited electrons.
Extrinsic Semiconductors
Extrinsic Semiconductors, on the other hand, are intrinsic semiconductors combined with other materials, such as boron or arsenic. This alters the natural behaviors or characteristics of the intrinsic semiconductors. In other words, when intrinsic semiconductors are doped, what you get are extrinsic semiconductors.
There are two types of extrinsic semiconductors, depending on the type of material that is added. The first type is the n-type semiconductors, which result from the addition of atoms that ve an extra electron. N-type means negative because of the extra electrons, which are typically from group V elements like phosphorus and arsenic. The other is the p-type semiconductors.
They are doped with elements like boron and gallium, which have only three electrons in the outer shell. This means that they are one electron short, unlike the n-type semiconductors. The resulting positive charge earned them the name p-type semiconductors.
Doping in Semiconductor Production
Doping is commonly used in semiconductor production to introduce impurities into an intrinsic semiconductor so as to modulate its electrical, optical, and structural properties. As explained above, doping silicon semiconductors produce the P-type and N-type silicon semiconductors. Generally, doped or extrinsic semiconductors may exhibit better electric conductivities than intrinsic semiconductors, in which case, they are called degenerate. Let’s dive deeper into the differences between the two.
P-Type Semiconductor
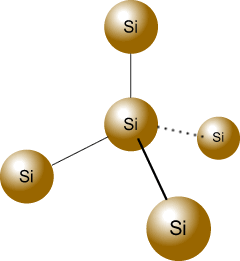
Instead of increasing the negative charge in the crystal lattice, the dopant, in this case increases the positive charge. Group III elements like boron or gallium serve as the dopant here. These elements have only 3 electrons in their outer shell, and when it mixes with the silicon crystal lattice, they form holes in the valence band of silicon atoms. Hence, the electrons in the valence band can roam freely, such that the hole moves in the opposite direction to the movement of the electrons.
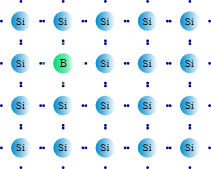
Since the electrons of the dopant atom are fixed in the lattice, only the positive charges can move around, giving rise to a net positive charge within the silicon-boron lattice. The positive holes earned these semiconductors their name, P-type semiconductors.
N-Type Semiconductors
N-type semiconductors contain dopants that have extra conduction electrons to the host material. A good example is doping silicon with phosphorus. Here, there’s an excess of electron charge carriers. This is because the doping phosphorus atom has one more valence electron than the host silicon atoms.
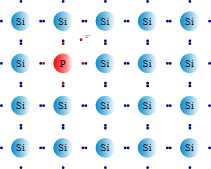
A silicon atom has 4 electrons bound tightly to its outer orbit. Phosphorus has 5 electrons instead. When combined, there’s a free electron from phosphorus. Since the electron carries a negative charge, this semiconductor is categorized as n-type.
Conclusion
The two types of silicon semiconductors are P-type and N-type semiconductors. These extrinsic semiconductors possess enhanced properties, which make them very useful in the electronic manufacturing industry.
Thank you for reading our article and we hope that it can help you better understand silicon semiconductors. If you want to know more about semiconductor materials, we would like to advise you to visit Stanford Advanced Materials (SAM) for more information.




