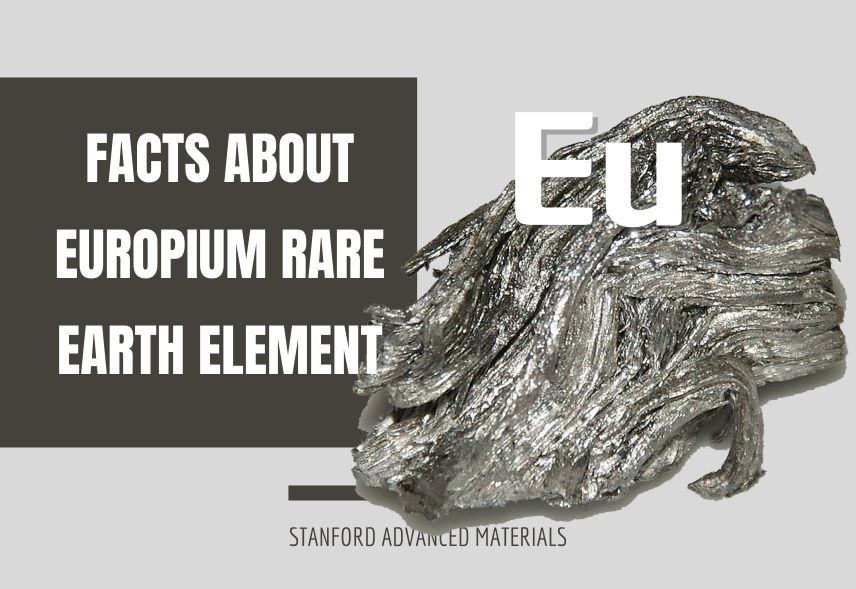
Europium is a chemical element that belongs to the Lanthanide series. This means it is a rare-earth element. It is distinct for being the most reactive, the rarest, the least dense, and the softest rare-earth element. It is so reactive that it is stored beneath a non-reactive fluid.
Europium is named after Europe. Paul-Émile Lecoq de Boisbaudran, a French chemist, discovered this rare-earth metal by extracting it from samarium-gadolinium concentrates in 1890. However, Eugene-Anatole Demarcay, a French chemist as well, was the first person to isolate Europium in its pure form. Europium is derived from bastnasite and monazite. It can also be found in the sun and stars.
Europium has two naturally occurring isotopes. The more abundant of the two is represented as 151 Eu while the less abundant is represented as 153 Eu. In addition to the two naturally occurring isotopes mentioned above, Europium has 35 radioisotopes.

Properties
Most rare-earth metals have similar chemical properties. Europium is not an exception to the rule. Despite these similarities that rare-earth elements have, Europium has many properties that distinguish it from other rare-earth elements.
Europium is a ductile, lustrous rare-earth element with a silvery appearance. It is phosphorescent, giving off a red glow. Europium loses its characteristic luster very easily to oxidation.
Europium’s periodicity is responsible for its properties. On the periodic table, Europium belongs to period 6, f-block. Europium has a body-centered cubic lattice system and a standard atomic weight of 151.964. It has two oxidation states which are +2 and +3. +3 is the more stable of the two states. It is also more common than the +2 state.
Furthermore, this rare-earth metal is solid at room temperature. Its melting point is 1099 K, and Its boiling point is 1802 K.
Europium is the least dense rare-earth metal. Its density is 5.264 g/cm3 when it is solid. However, when it is liquid, its density decreases to 5.13 g/cm3.
Europium has many chemical reactions. It oxidizes in the air at a fast rate and is easily combustible. It is soluble in dilute H2SO4 and reacts with all halogens. When it reacts with water, it forms europium hydroxide.
Europium is one of the softest rare-earth metals. It is soft enough to be cut through with a knife. It is paramagnetic above 90 K but is antiferromagnetic below 90 K. It is also electropositive, and its electronegativity on the Pauling scale is 1.2.
Applications
Europium has several applications in the world today. These applications are owing to both its physical and chemical properties. Like most other rare-earth metals, it is usually used in the form of a compound or an alloy. Here are some of the applications of Europium:
Television Screens
Europium is used in the production of television screens. The property of Europium that allows it to be used in the manufacture of television screens is its phosphorescence. In its oxidized form, Europium is used as a red phosphor for color television screens.
Computer Monitors
Like color television screens, Europium can also be used in computer screens. The phosphorescent property of Europium is again responsible for this application.
Dopant
Europium is used as a dopant for semiconductors. The doped material can be used for laser technology.
Banknotes
Europium can be used in printing banknotes. Europium present in banknotes can be used to detect counterfeit notes. Europium will usually have a red glow under UV light. However, counterfeit banknotes will not have this glow under UV light.
Thin-Film Coating
Europium sputtering targets and europium evaporation materials are used in deposition processes including semiconductor deposition, chemical vapor deposition (CVD) and physical vapor deposition (PVD).
Alloys
Although Europium’s status as a superconductor has not been fully established, it is said to be a superconductor. It is used in producing alloys for materials, equipment, or tools that require a superconductor.
Medicine
Europium compounds can be added to antibodies. These antibodies react with antigens to form antigen-antibody complexes. The Europium compounds give off a red glow that can be used to quantify the antibodies in body fluids.
Nuclear Reactors
Europium has a high neutron capture cross-section. This allows it to be used as a control rod in nuclear reactors
Memory Chips
Europium compounds are used to produce quantum memory chips. The uni9t for storing information in quantum chips is qubits. Research and works are still ongoing towards developing quantum memory chips.
Conclusion
Europium is one of the most expensive rare-earth metals, and it is less used than the other rare-earth metals. However, the profits of its application generally outweigh the cost of its extraction. It is especially important in industries that use phosphorescence.
If you want to know more about rare earth elements, we would like to advise you to visit Stanford Advanced Materials (SAM) for more information.